Unified User Experience: All your work in one system
Research Products
Research Products
Research Products
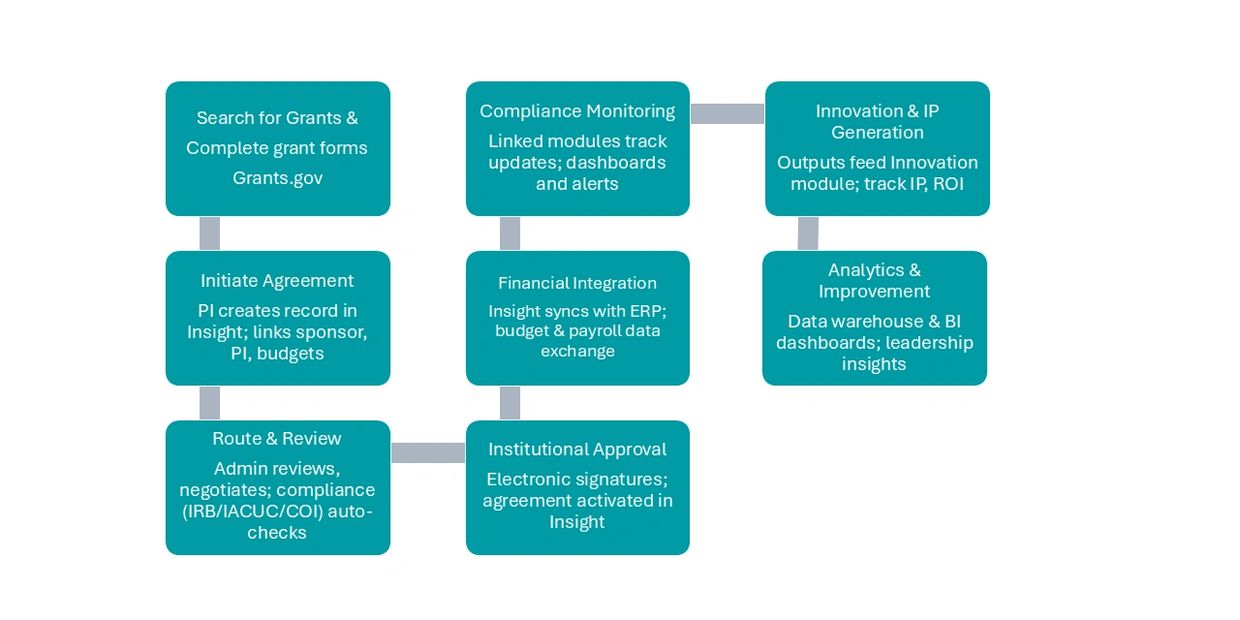
Agreements
- Encompasses new submissions for grants, contracts, clinical trial agreements, internal funding and more with electronic workflows from faculty to administrative review.
- Transitions requested funding to post award setup, management and financials for a seamless user experience and full view of the agreement lifecycle and amendments.
- Real-time integration into ERP systems to eliminate redundancies, improve data quality and improve efficiencies.
- Once awarded, projects can be tracked with financial reporting capabilities and other functions to help manage sponsor commitments.
Research Products
Research Products
Agreements (cont.)
- Once awarded, projects can be tracked with financial reporting capabilities and other functions to help manage sponsor commitments.
- Integrated functions to support the award lifecycle including award renewals, Other Support information, progress reports, residual balance transfers and more.
- Ability to create sub-award, consortium, clinical site, data use and other templates that are compliant with applicable regulation to help streamline the contracting process.
- Facilitates Innovation contracting and financials.
- Integrates with Space Management, Humans, Animals, Biosafety, Disclosures, Effort Reporting, and Innovation modules.
Research Products
Compliance Products
Personnel
- Tracks how a person is getting paid from multiple funding sources, including grants.
- Facilitates salary management on sponsored projects to see a person’s portion of salary dedicated to projects as it spans fiscal, calendar or school year.
- Integrates with Effort Reporting module.
Space Management
- Tracks all research locations, including what projects are assigned to the location, equipment located there and other important data points to conduct space surveys, equipment audits, density reporting and F&A rate negotiations.
- Integrates with your enterprise's capital asset management system.
- Integrates with Agreements module.
Compliance Products
Compliance Products
Compliance Products
Animal Studies/IACUC
- Features forms, document management, staff management and workflow capabilities to help organizations with their IACUC programs.
- Integrates with Agreements, Biosafety and Meeting Management modules.
Human Studies
- Allows any institution to manage its processes relating to institutional review board functions – protocol creation, forms management, initial review, amendments, other event workflows and more.
- Ability to configure ancillary reviews for review of a protocol.
- External integration to training databases like CITI, clinical trial management systems, and more.
- Integrates with Agreements, Disclosures and Meeting Management modules.
Biosafety
- Biohazard management to oversee, review all recombinant DNA (rDNA), infectious agents, human and non-human primate materials, and biological toxins.
- Integrates with Agreements, Humans, Animals and Meeting Management.
Compliance Products
Disclosures
- Simplifies Conflict of Interest/Commitment (COI) compliance process by placing the forms at the fingertips of researchers.
- Embedded into the landing page and logic-based forms, checklists, and help texts to guide users through the compliance form sets.
- Facilitates annual disclosure processes.
- Facilitates disclosures by agreement/protocol.
- Alerts users on comments, upcoming form due dates, and other feedback.
- Integrates with Agreements and Humans modules.
Effort Reporting
- Designed to meet Federal and sponsor effort reporting requirements.
- Provides queries, workflows, investigator hierarchy and certification capabilities.
- Integrates with your enterprise's Payroll management solution.
- Integrates with Agreements and Personnel modules.
Meeting Management
- Facilitates IRB, IACUC, and IBC reviews.
- Supports protocol and registration review processes for determination by the relevant boards.
- Facilitates protocol reviews and approvals by committee members.
- Integrates with the Human Studies, Animal Studies, and Biosafety modules.
Compliance Products
TEFRA
- For those organizations filing Medicare Cost Reports, the TEFRA solution provides a platform for clinicians to certify clinical time spent that helps drive cost reimbursement.
- Integrates with Payroll.
Export Control
- Designed to comply with laws and regulations that govern the export of sensitive technology, goods, services and information from one country to another.
- Aimed at protecting national security, support foreign policy objectives and ensure compliance with international agreements.
- Conducts exclusion checks, evaluation of Research Projects and classification of Equipment, Biological Samples, Software or Technology.
- Assists Institutional Export Control Officers to check numerous government lists of restricted and denied parties and dynamically screen all queries after initial checks by adding them to watch lists.
Innovation/Tech Transfer Products
Innovation/Tech Transfer Products
Innovation/Tech Transfer Products
Outcomes from research projects or other university inventions are potential assets to review and determine patentability. It is also important to know the sources of funding for inventions and potential federal reporting responsibilities.
With our Innovation module, you can track and collaborate on the transformation of research outcomes into documented disclosures, IP filings, legal billing, federal compliance, licensing, invoicing and more.
Innovation/Tech Transfer Products
Innovation/Tech Transfer Products
Contracting
- Contracts management system to support negotiations, signatures and executions.
- Tracks terms of agreement and generates necessary invoices and obligations including back and ongoing patent expenses.
- Through workflow electronically captures information to process distribution of licensing income to inventors, constituents and your organization.
- Financial overview providing Revenue vs. Net Expenses.
- Integrates with Inventions, Intellectual Property and Financials functions.
Financials
- Processing of legal bills and expenses.
- Generates invoices and tracks relationship between payment, expense reimbursements and distributions.
Innovation/Tech Transfer Products
System Administration Products
Invention Disclosure
- System for the collection of new innovator ideas that could lead to IP protection and licensing.
- Integrates with the Agreements module to define relationship of invention to funding sources for NIST and other reporting.
- Allows for input, tracking and reporting on internal business processes in reviewing new inventions including Prior Art Searches, Coversheet filings and more.
- Captures NIST information and inventor contribution for full invention reporting.
- Integrates with Intellectual Property, Contracting and Financials functions.
Intellectual Property
- Patent prosecution tracking system for managing intellectual property.
- Financial reports are generated providing a clear understanding of reimbursed/unreimbursed expenses related to each IP record.
- Integrates with Inventions, Contracting and Financials functions.
System Administration Products
System Administration Products
System Administration Products
Core modules used to support all other modules and your organization's information and security.
System Administration Products
Administration
- Designed for forms engine, workflows, notifications, templates, and communication management.
- Supports sponsor/outside organization management and workflow review.
- Management of department/unit hierarchy and other related institution information.
System Administration Products
Manage Profiles and Security
- Supports all areas of your research community to maintain security and configuration of the modules.
- Allows the departments to maintain their contacts and security.
- Allows users to view or updated profile demographic information.
- Facilitates electronic annual review of security for audit certification.